Simulation of Yield Strength of Aluminum Alloy 356
Purpose: Learn to calculate yield strength including intrinsic, solid solution, and precipitation strengthening of aluminum alloy 356 during the process of ageing
Module: PanEvolution
Thermodynamic and Mobility Database: AlMgSi.tdb
Kinetic Parameters Database: AA3xx.kdb
Batch file: Example_#3.6.pbfx
Calculation Procedures:
-
Create a workspace and select the PanEvolution module following Pandat User's Guide: Workspace ;
-
Load AlMgSi.tdb following the procedure in Pandat User's Guide: Load Database ;
-
Click on PanEvolution/PanPrecipitation on the menu bar and select "Load KDB or EKDB", then select the AA3xx.kdb;
-
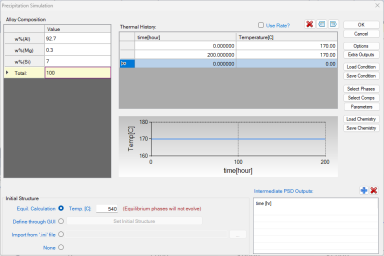
Click on the menu "PanEvolution/PanPrecipitation → Precipitation Simulation", and set up the calculation condition as shown in Figure 1. Note that the “Equilibrium Calculation” button under Initial Structure (bottom) should be checked and the solution temperature (540 °C in this case) should be given;
Post Calculation Operation:
-
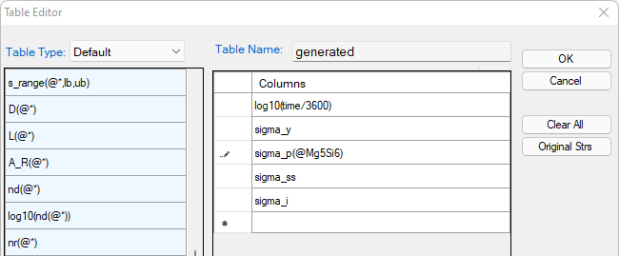
Create a new table as shown in Figure 2, and select all the columns in the newly created table to create a plot;
-
Right Click on the Table node below the Graph and choose "Import Table from File", import table: AA356-ys_exp.txt; Note that the extension of a data file can be either txt or dat;
-
Plot the experimental data in the corresponding plot by drag in the logt as x-axis and then press Ctrl and drag in the experimental data ys as the y-axis, then repeat it to add experimental data of ysp, ysi and yss;
-
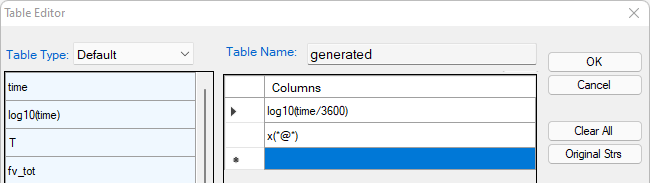
Create a new table as shown in Figure 4, select log10(time/3600) as x-axis, and x%(Mg@_Fcc) and x%(Si@_Fcc) as y-axis to create a plot as shown in Figure 5;
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
-
Add legend for graph following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
Figure 3: A comparison of the calculated yield strength (lines) with the experimental data (symbols)
Information obtained from this calculation:
-
shows a comparison of the calculated yield strength (lines) with the experimental data (symbols). The orange line (symbols) represents the intrinsic contribution; the green line (symbols) represents the solid solution strengthening; the red line (symbols) represents the precipitation strengthening; and the blue line (symbols) is the total from all three contributions;
-
It is clearly seen from Figure 3 and Figure 5 that with the formation of Mg5Si6 precipitate, the solubility of Mg and Si in Fcc decreases and solution strengthening also decreases.